Co-Innovative Bespoke CRO Platform
Bringing Ideas to Therapeutic Impacts
Drug Discovery with AI, In Vitro & In Vivo Bespoke Models and Global Collaboration
Welcome to TME Scientific, your one-stop biomodelling and CRO service provider.
TME (Therapeutic Modelling & Experimentation) is at the forefront of AI-driven drug discovery, offering advanced CRO services that integrate humanized modeling, preclinical and translational research, and AI-powered study design. As a collaborative platform, TME connects academic researchers, CROs, CMOs, CDMOs, and biotech innovators, accelerating the development of life-saving therapies.
Our Capabilities at a Glance

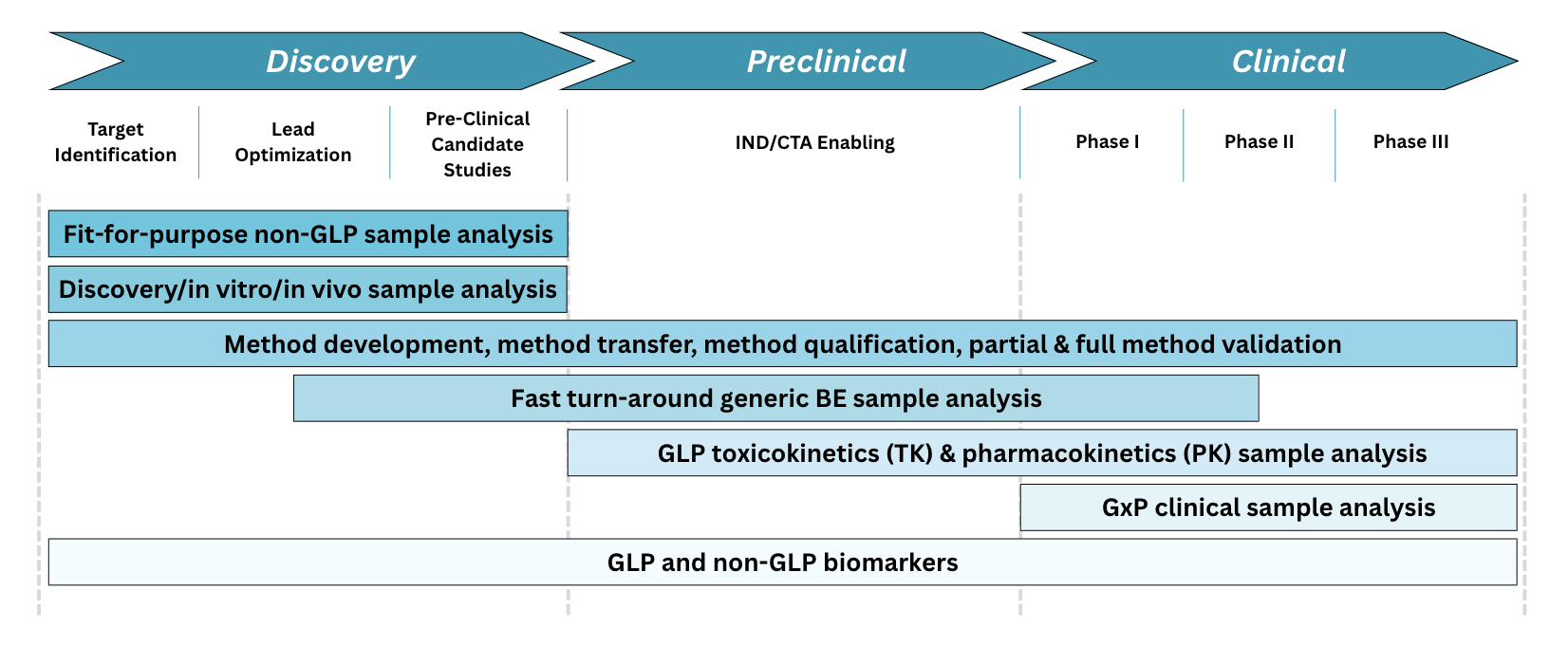
From Discovery to the Clinic: The Drug Development Journey
Bringing a new therapy to market is a complex, multi-phase process that begins with target discovery and ends with regulatory approval. Each stage - discovery, preclinical, and clinical - builds on the last, generating critical data to evaluate safety, efficacy, and viability. Our diagram below outlines this pathway, highlighting key milestones and decision points along the way.
GLP vs. Non-GLP Toxicology Studies
In drug development, understanding when to apply Good Laboratory Practice (GLP) guidelines versus non-GLP approaches can save time, reduce costs, and improve decision-making. While GLP studies are essential for regulatory submissions and ensuring data integrity, non-GLP studies play a critical role in early-stage research by offering flexibility and speed without compromising scientific quality. Knowing which path to take, and when, can accelerate progress and optimize resources.
GLP (Good Laboratory Practices) ensures the integrity, reliability, and regulatory acceptance of nonclinical studies, especially those required for IND submissions. It mandates structured processes, documentation, qualified personnel, and strict quality control.
Non-GLP studies are acceptable for early-stage research like in vitro toxicology, ADME, genotoxicity, and safety pharmacology. They’re faster, less costly, and useful for early go/no-go decisions, though they still require high-quality, reviewable data.
Key differences:
GLP: Required for regulatory submission; strict processes and documentation.
Non-GLP: Suitable for early exploration; faster and cheaper, but still must be scientifically sound.