Cell-Based Immunoassays
Drug discovery is a multidisciplinary process aimed at identifying potential new medicines. It involves identifying screening hits, optimizing them through medicinal chemistry to enhance their properties, and validating targets for efficacy and safety. At TME Scientific, we offer comprehensive drug discovery services, including target identification and validation, lead identification and candidate optimization, and specialized cell model services. Explore our offerings to enhance and streamline your drug discovery journey.
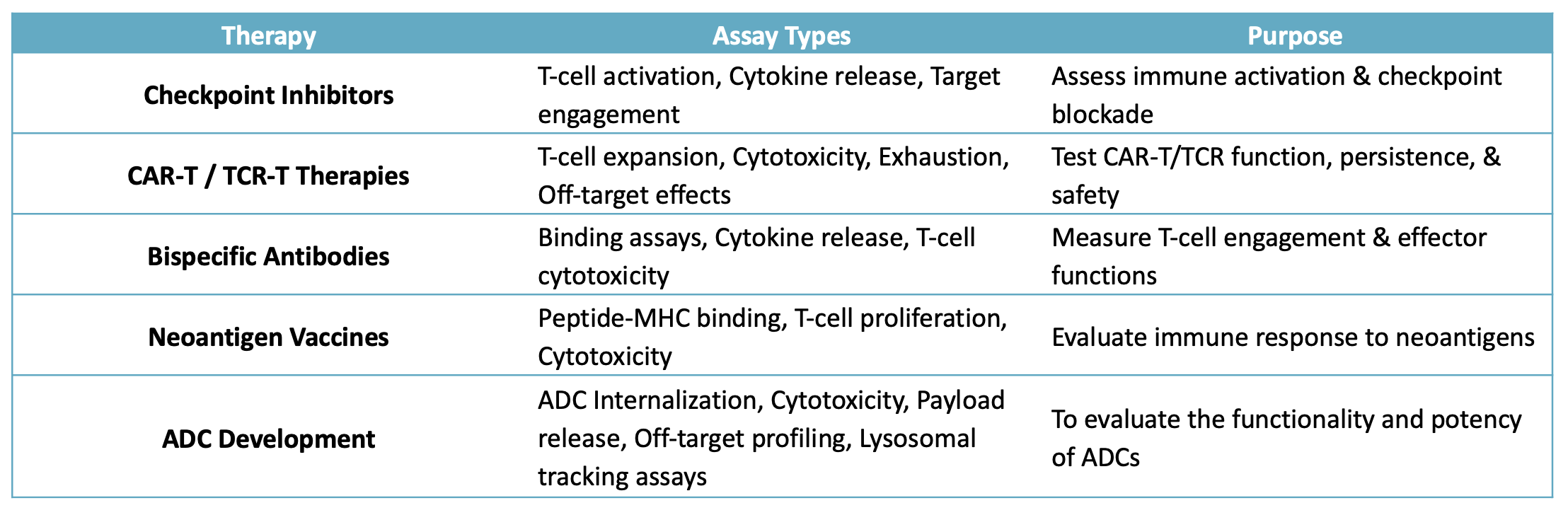
Summary Table of Key In Vitro Assays for Immunotherapies:
Key Functional Assays to Assess Cancer Immunotherapies:
Take a moment to view our services