The Cytokine Release Assay is a critical assay for validating neoantigens, assessing their ability to activate T-cells, and inducing immune responses. This assay helps determine whether a neoantigen can stimulate T-cells to release cytokines, indicating a functional immune response.
Cytokine Release Assay for Neoantigen Validation
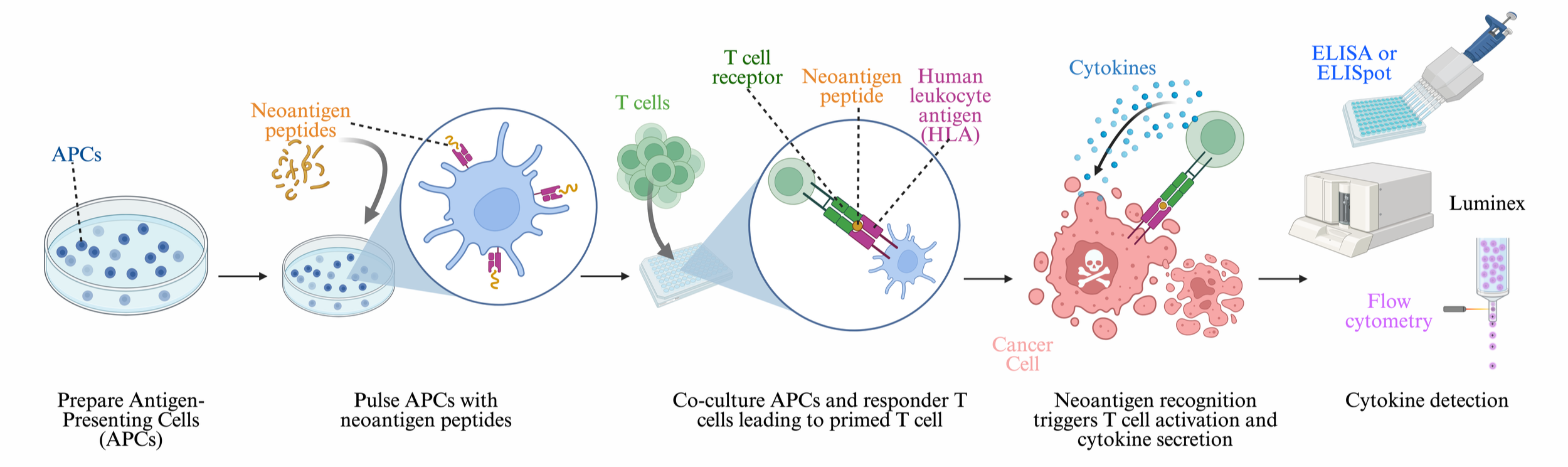
Principle of Cytokine Release Assay
The Cytokine Release Assay assesses T-cell activation by measuring cytokine secretion (IFN-γ, IL-2, TNF-α).
When T-cells recognize a neoantigen presented by antigen-presenting cells, they become activated and release cytokines.
These cytokines can be detected using ELISPOT, ELISA, Luminex, or flow cytometry.
Cytokine Detection Methods
ELISA → measures IFN-γ, IL-2, TNF-α, etc.
ELISpot Assay → detects single T-cells secreting cytokines (high sensitivity).
Multiplex Bead-Based Assays (Luminex, MSD) → quantifies multiple cytokines simultaneously.
Intracellular Cytokine Staining (ICS) + Flow Cytometry → identifies cytokine-producing CD8+/CD4+ T-cells.
Add-On Services
Evaluation of additional cytokine release, T-cell activation, proteomics, and transcriptomics.
Assay Key Components
A. T-Cells (Responder Cells)
Autologous peripheral blood mononuclear cells (from patient) or tumor-infiltrating lymphocytes.
Can also use CD8+ or CD4+ T-cell-enriched fractions.
B. Antigen-Presenting Cells
Dendritic Cells (derived from monocytes) or autologous PBMCs.
Can also use T2 cells (HLA-A2+ TAP-deficient cells) for MHC class I-restricted peptides.
C. Neoantigen Peptides
Synthetic peptides (25-30 amino acids long) covering predicted neoepitopes.
Can be loaded onto antigen-presenting cells for presentation to T-cells.
D. Co-Culture System
Antigen-presenting cells are pulsed with neoantigen peptides and incubated before adding T-cells.
T-cells and antigen-presenting cells are co-cultured in a 96-well plate. This ensures effective antigen presentation and T-cell stimulation.